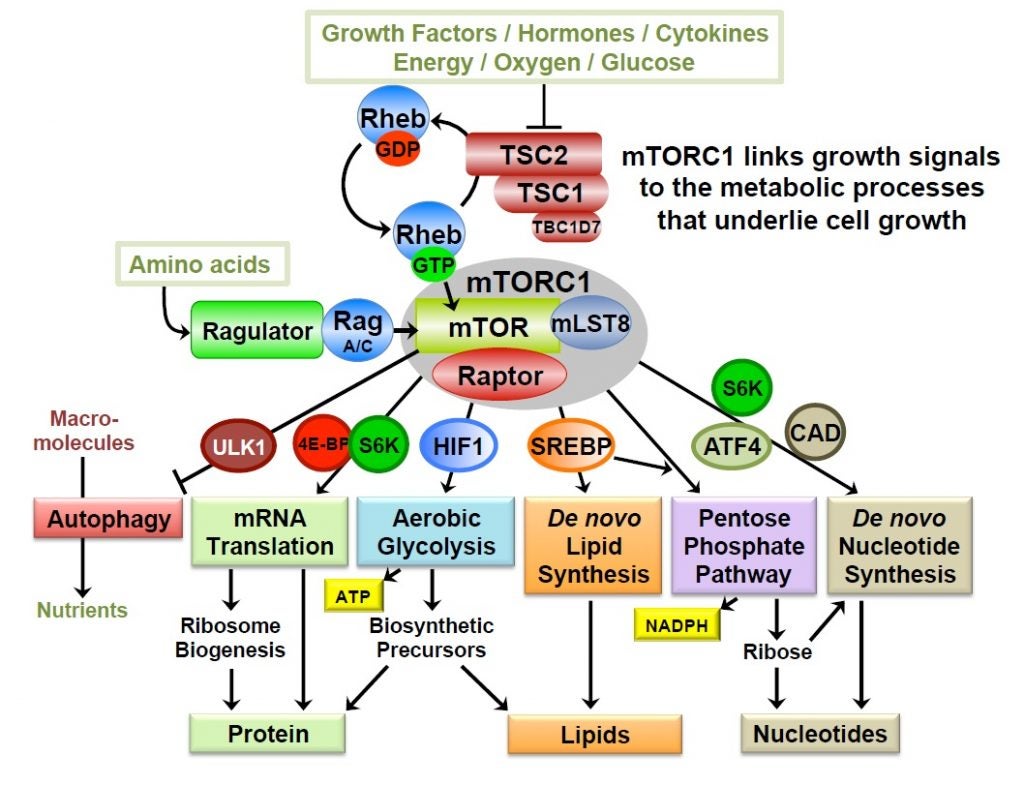
In studies initiated through a variety of unbiased omics approaches, the Manning lab has found that the primary function of the PI3K-mTOR network is to control cellular metabolism. The network serves to link growth signals to mechanisms controlling the key anabolic processes that underlie growth. Activation of mTOR complex 1 (mTORC1) in response to nutrients and other growth signals promotes the conversion of nutrients into biomass through downstream transcriptional and post-translational effects on major metabolic enzymes, such as those influencing protein, lipid and nucleotide synthesis. Through these molecular mechanisms, the network controls cell and tissue growth, as well as integrated systemic metabolism. The lab is particularly interested in cancer metabolism and how the common dysregulation of PI3K and mTORC1 in human tumors alters the metabolism of tumor cells and distinguishes them from their cells of origin.
Relevant Publications
Soflaee MH, Kesavan R, Sahu U, Tasdogan A, Villa E, Djabari Z, Cai F, Tran DH, Vu HS, Ali ES, Rion H, O’Hara BP, Kelekar S, Hallett JH, Martin M, Mathews TP, Gao P, Asara JM, Manning BD, Ben-Sahra I, Hoxhaj G. Purine nucleotide depletion prompts cell migration by stimulating the serine synthesis pathway. Nat Commun. 2022 May 16;13(1):2698. doi: 10.1038/s41467-022-30362-z. PMID: 35577785; PMCID: PMC9110385.
Byles V, Cormerais Y, Kalafut K, Barrera V, Hughes Hallett JE, Sui SH, Asara JM, Adams CM, Hoxhaj G, Ben-Sahra I, Manning BD. Hepatic mTORC1 signaling activates ATF4 as part of its metabolic response to feeding and insulin. Mol Metab. 2021 Nov;53:101309. doi: 10.1016/j.molmet.2021.101309. Epub 2021 Jul 23. PMID: 34303878; PMCID: PMC8368025.
Torrence ME, MacArthur MR, Hosios AM, Valvezan AJ, Asara JM, Mitchell JR, Manning BD. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. Elife. 2021 Mar 1;10:e63326. doi: 10.7554/eLife.63326. PMID: 33646118; PMCID: PMC7997658.
Hoxhaj G, Ben-Sahra I, Lockwood SE, Timson RC, Byles V, Henning GT, Gao P, Selfors LM, Asara JM, Manning BD. Direct stimulation of NADP+ synthesis through Akt-mediated phosphorylation of NAD kinase. Science. 2019 Mar 8;363(6431):1088-1092. doi: 10.1126/science.aau3903. PMID: 30846598; PMCID: PMC7261235
Valvezan AJ, Turner M, Belaid A, Lam HC, Miller SK, McNamara MC, Baglini C, Housden BE, Perrimon N, Kwiatkowski DJ, Asara JM, Henske EP, Manning BD. mTORC1 Couples Nucleotide Synthesis to Nucleotide Demand Resulting in a Targetable Metabolic Vulnerability. Cancer Cell. 2017; pii: S1535-6108(17)30417-8. PMID: 29056426
Hoxhaj G, Hughes-Hallett J, Timson RC, Ilagan E, Yuan M, Asara JM, Ben-Sahra I, Manning BD. The mTORC1 Signaling Network Senses Changes in Cellular Purine Nucleotide Levels. Cell Rep. 2017; 21(5):1331-1346. PMID: 29091770
Ben-Sahra I, Manning BD. mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol. 2017; 45:72-82. PMID: 28411448
Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, Hoxhaj G, Saghatelian A, Shaw RJ, Manning BD. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017; 25(2):463-471. PMID: 28089566
Ricoult SJ, Dibble CC, Asara JM, Manning BD. SREBP regulates the expression and metabolic functions of wild-type and oncogenic IDH1. Mol Cell Biol. 2016; 36(18):2384-95. PMID: 27354064
Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016; 351(6274):728-33. PMID: 26912861
Ricoult SJ, Yecies JL, Ben-Sahra I, Manning BD. Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene. 2016; 35(10):1250-60. PMID: 26028026
Zhang Y, Nicholatos J, Dreier JR, Ricoult SJ, Widenmaier SB, Hotamisligil GS, Kwiatkowski DJ, Manning BD. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature. 2014; 513(7518):440-3. PMID: 25043031
Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013; 339(6125):1323-8. PMID: 23429703
Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, Manning BD. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011; 14(1):21-32. PMID: 21723501
Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010; 39(2):171-83. PMID: 20670887
Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009; 4(7):e6189. PMID: 19593385