Finding links physics of granular systems to biology of asthma and embryonic development
For immediate release: April 2, 2018
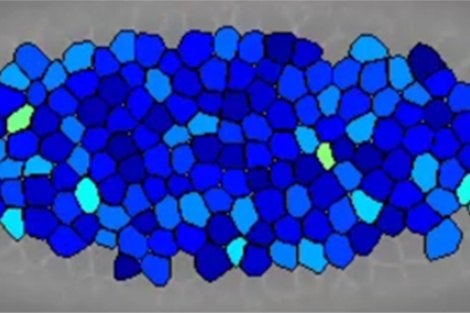
Boston, MA – Cells comprising a tissue can pack into disorderly geometries much as do grains of sand in a sandcastle. In doing so they can freeze into a fixed shape—as in a sandcastle—or flow like sand poured from a beach bucket. The finding, reported by researchers at Harvard T.H. Chan School of Public Health, Northeastern University, and MIT, provides insights into organ formation in an embryo, healing of a wound, and even invasion of cells into surrounding tissue, as occurs in cancer.
“This finding makes a deep connection between the physics of inert granular matter such as sand and the geometry of multicellular living systems,” said lead author Lior Atia, a postdoctoral fellow in the laboratory of Jeffrey Fredberg, professor of bioengineering and physiology at Harvard Chan School. “Due to the nature of how a cell nestles among its immediate neighbors, a scientist can now look at cell shapes and make a reasonable guess as to why, and how fast, those cells will migrate, remodel, or invade surrounding tissues.”
The study appears online April 2, 2018 in Nature Physics.
Previous work by Fredberg and colleagues had documented the importance of collective cellular behavior in asthma, showing that cells comprising epithelial tissues—which line the surfaces of all organs throughout the body— can unjam and flow like a fluid, or jam and freeze like a solid.
In the new study, the researchers explored the role of cell shape in two vastly different types of epithelial cells—human bronchial epithelial cells grown in the lab and cells within the living embryo of the fruit fly—and observed them as they matured over time.
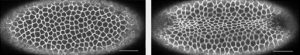
As cells jammed or unjammed, cell shapes changed in a systematic manner in both systems, thus suggesting a common physical basis. Cell shapes became progressively less elongated and less variable as they jammed, and more elongated and more variable as they unjammed. Much like inert granular matter, these cells interacted with their nearest neighbors to form a “disordered collective” that transitioned between solid-like and fluid-like states.
According to the researchers, these findings suggest that differences in shape from cell-to-cell within a tissue are key to their ability to jam and unjam—and that this process appears to drive biological events including embryonic development, wound healing, and, potentially, cancer cell invasion.
This insight into cell behavior could help researchers better understand cell jamming and unjamming, which could lead to potential treatments for conditions such as developmental abnormalities, asthma, and cancer.
Other Harvard Chan authors include Yasha Sharma, Jennifer A. Mitchel, Bomi Gweon, Stephan Koehler, Stephen J. DeCamp, Bo Lan, Jae Hun Kim, Rebecca Hirsch, Jin-Ah Park, and James P. Butler.
Support for the study came from: Harvard Catalyst Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers, National Cancer Institute grant number 1U01CA202123, National Heart Lung and Blood Institute grant numbers R01HL107561, PO1HL120839, and T32 HL007118, and the National Research Foundation of Korea grant number NRF-2014R1A6A3A04059713.
“Geometrical constraints during epithelial jamming,” Lior Atia, Dapeng Bi, Yasha Sharma, Jennifer A. Mitchel, Bomi Gweon, Stephan Koehler, Stephen J. DeCamp, Bo Lan, Jae Hun Kim, Rebecca Hirsch, Adrian F. Pegoraro, Kyu Ha Lee, Jacqueline R. Starr, David A. Weitz, Adam C. Martin, Jin-Ah Park, James P. Butler, Jeffrey J. Fredberg, Nature Physics, online April 2, 2018, doi: 10.1038/s41567-018-0089-9
Visit the Harvard Chan School website for the latest news, press releases, and multimedia offerings.
For more information:
Todd Datz
tdatz@hsph.harvard.edu
617.432.8413
Images courtesy Fredberg lab
###
Harvard T.H. Chan School of Public Health brings together dedicated experts from many disciplines to educate new generations of global health leaders and produce powerful ideas that improve the lives and health of people everywhere. As a community of leading scientists, educators, and students, we work together to take innovative ideas from the laboratory to people’s lives—not only making scientific breakthroughs, but also working to change individual behaviors, public policies, and health care practices. Each year, more than 400 faculty members at Harvard Chan School teach 1,000-plus full-time students from around the world and train thousands more through online and executive education courses. Founded in 1913 as the Harvard-MIT School of Health Officers, the School is recognized as America’s oldest professional training program in public health.