My research focuses on the role of airway epithelium in the lung. The airway epithelium is the first line of defense against external stimuli. Airway epithelial cells lining on the airway epithelium contribute to homeostasis in the lung, but when they are exposed to excessive biochemical or physical stimuli, the normal defense mechanism turns into the progression of various pathophysiologic conditions. Airway epithelial cells also play important roles in innate and adaptive immunities and inflammation.
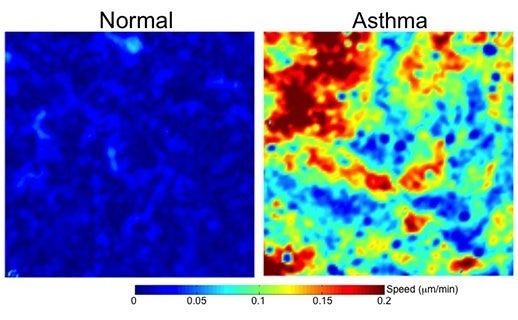
Much of the morbidity and mortality associated with persistent asthma is attributable to progressive and irreversible remodeling of the airway wall. Most theories concerning airway remodeling argue that the remodeling process is triggered in response to inflammatory mediators and cytokines.
We hypothesize that airway remodeling is triggered in response to the mechanical stress imposed on the airway epithelium during bronchoconstriction.
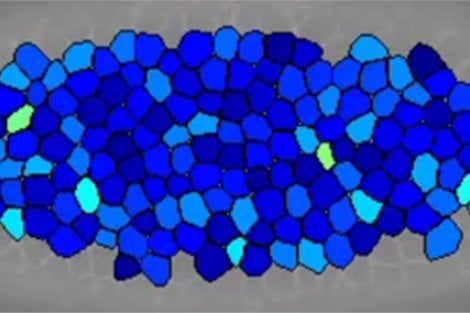
We use a compressive in vitro model system (Figure 1). In this system, primary human bronchial epithelial cells are grown in an air-liquid interface (ALI) culture and subjected to compressive stress, which is modeled through the application of a transepithelial pressure gradient at a magnitude of 20 to 30 cm H2O. This magnitude of stress is similar to the magnitude that bronchoconstriction imposes on the airway epithelium, and is significantly higher than the magnitude experienced during normal breathing. The compressive in vitro system recapitulates many aspects of airway remodeling in the absence of inflammatory cells (Putting the Squeeze on Airway Epithelia, Park et al. Physiology. 2015).
The findings using the compressive in vitro system have already led to experiments in humans showing that methacholine-induced bronchoconstriction, in the absence of eosinophilic inflammation, can lead to collagen deposition under the epithelial monolayer, enhanced expression of TGF-*, and increased numbers of goblet cells - key aspects of airway remodeling. These experiments provide in vivo validation of our experimental approach.
A full understanding of the asthmatic response requires many different models; in vitro compressive model system highlights and isolates the effects of mechanical stress on airway epithelial cells. We also use mouse asthma models to verify the physiological effects of target molecules and to identify further mechanisms.
International Research Visiting Fellowship (The University of Newcastle, Australia)2017
The University of Newcastle, Australia
Ann Woolcock Memorial Award (American Thoracic Society)2014
American Thoracic Society
Parker B. Francis Fellowship2013-2016
Francis Family Foundation
Scientist Development Grant, AHA2013-2016
American Heart Association