February 17, 2023 – Researchers at Harvard T.H. Chan School of Public Health have developed a new, highly adaptable vaccine platform that could potentially be a powerful tool in the fight against viral pathogens including influenza, HIV, and SARS-CoV-2.
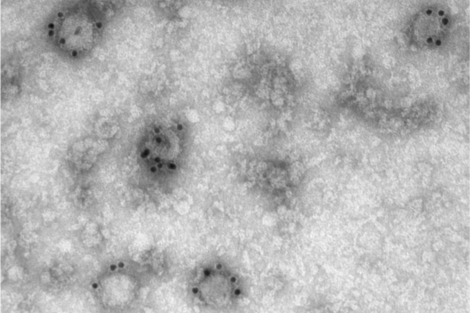
Postdoctoral fellow Sengjin Choi; Quan Lu, Cecil K. and Philip Drinker Professor of Environmental Physiology; and others in Lu’s lab created a platform that makes use of tiny particles found in the body called extracellular vesicles, or EVs, which transport various molecules among cells. Working with colleagues at the University of Nebraska, the researchers repurposed a particular type of EVs—called WW domain-activated EVs (WAEVs)—to create the new vaccine platform.
The study was published January 27 in Science Advances.
One common way vaccines work is by using viral proteins as antigens—markers that the immune system can recognize—to stimulate protection against an infection. Antigens are typically proteins or sugars found on the outside of cells or viruses, and each antigen has a unique shape that the immune system can “read” to know whether or not it belongs in the body. If the body recognizes antigens that are dangerous—part of an intruding virus—it will produce antibodies that specifically fit and bind onto those antigens, and neutralize and eventually destroy the virus.
The problem with some vaccines, however, is that the structure of the viral antigens they use may not be exactly the structure needed to kick the immune system into high gear. For instance, certain viruses, such as those that cause the flu, HIV/AIDS, and COVID-19, have antigens that are embedded in a “lipid bilayer”—a double layer of lipids—that coats the virus. Because most current vaccines deliver antigens without a lipid bilayer, the structure of the resulting antibodies may make it more difficult for them to attach to and neutralize the targeted virus.
In the new research, scientists inserted flu or HIV antigens into EVs—which, according to Lu, “are essentially squishy little balls encapsulated with a lipid bilayer,” making them an ideal medium to hold the viral protein antigens in the correct structure so that they can be effectively recognized by the immune system. Testing the EV packages in mouse models of the diseases, the researchers found that the mice that received the EV vaccines had higher antibody production and significantly longer survival rates than those that did not receive those vaccines.
“Our study showed that WAEVs can serve as a novel, highly adaptable vaccine platform that offers a potentially powerful tool in our fight against viral pathogens,” said Lu. “While this study was focused on flu and HIV viruses, the system can be used against other viruses such as SARS-CoV-2.”
Repurposing EVs for vaccine delivery has further public health advantages, Lu said. For example, current mRNA vaccines such as the ones developed for COVID-19 need to be stored in ultra-cold freezer conditions. Because vaccines using EVs are more stable, they only require refrigeration, therefore making it easier and less costly to deliver vaccines to low-resource areas.
Feature photo courtesy Quan Lu
Photo of Quan Lu: Kent Dayton