Aging is a universal trait that is observed across the evolutionary spectrum. From a public health perspective, aging is also the critical risk factor for a variety of human pathologies, including neurodegenerative diseases such as Alzheimer’s, many forms of cancer and metabolic disease/type II diabetes, which have become much more prevalent in the elderly. Determining the causal underlying cellular and molecular processes that deteriorate with age and lead to increased disease susceptibility and frailty is critical if we are to meet the growing healthcare needs of aging human populations. Our lab is interested in understanding the molecular pathways underpinning the aging process, with the goal of using this knowledge to develop novel therapeutic strategies to treat age-onset disorders.
Why Aging is a Critical and Emerging Problem in Public Health:
The last 100 years has seen dramatic advances in the numbers of people living into old age, with the majority of this increase resulting from better public health. However, this very fact we are living longer has increased the numbers of elderly patients suffering with chronic age-onset diseases, often with multiple co-morbidities. Therefore, if the one of the greatest successes of public health in the last one hundred years was adding years to our lives, the next challenge is to make those added years healthy.
See video below about how aging research might yield a ‘longevity dividend’, with huge impact on society socially and economically with high potential for elevating the burden of disease in the elderly:
Video Credit: Professor Stuart Jay Olshansky, School of Public Health, University of Illinois
Why Study Aging in a Worm?
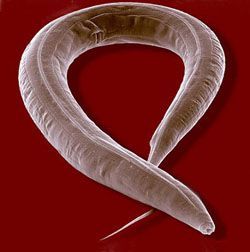
Part of our lab uses the nematode worm C. elegans to study the aging process – but what can the worm tell us about human age-related disease? Aging is a complex trait, and there is a wide variety in the rate at which different organisms senesce. This variety is even seen in closely related organisms – the brown rat lives 2 years, while the naked mole rat can live for 30. Some nematodes live 2 days while others survive for 10 years. Even within the same organism, different tissues age at different speeds and communication from one cell type can change the longevity of the entire organism. Therefore, to understand such a complex process, we need a manipulatable system that allows us to systematically untangle the molecular pathways involved.
C. elegans are 1.5mm long, transparent nematodes commonly found in soil across the globe. They live about 2 weeks, and during that short time they display obvious signs of aging, including reduced locomotion, reproductive decline, reduced stress resistance, sarcopenia and a collapse in various homeostatic mechanisms – even their skin wrinkles. This makes the nematode an ideal system to test the effects of an intervention on longevity; in a short space of time we can assess if a genetic or pharmacological intervention increases longevity. This is critical, since determining if an intervention that shortens lifespan does so by speeding aging up or inducing pathology is difficult – lifespan extension is clearer to interpret. The worm also comes equipped with a variety of tools that allow for easy genetic manipulation. Being able to rapidly and cost effectively test multiple interventions therefore helps us expedite discovery. Further, given C. elegans are transparent, we are able to analyze gene expression, protein localization and communication between distal tissue and cell types in vivo as an individual ages using fluorescence microscopy.
How does this relate to human health? The goal is certainly not to make a long-lived worm and stop there, but instead find evolutionarily conserved pathways that protect against age-related decline and pathology and translate them to treat human disease. Aging is a genetically mediated, heritable and malleable trait. During the course of evolution, organisms have developed mechanisms by which they can alter the rate at which they age in response to stress – in order to see out the hard times. A robust example of this is the increased longevity seen when energy availability is low. Strikingly, not only are animals with restricted energy intake long-lived, they are also resistant to multiple age-related pathologies. Therefore, by understanding the conserved molecular and genetic pathways that mediate this disease protection, we can apply what we learn from the simple nematode to human pathology. Supporting this approach, single gene mutations first shown to extend the lifespan in ‘the worm’, have now been shown to slow aging and protect against age-onset pathology in other model organisms such as fruit flies and mice, and have been linked to extreme longevity seen in centenarian populations. As Friedrich Nietzsche said: ‘You have evolved from worm to man, but much within you is still worm’…..



