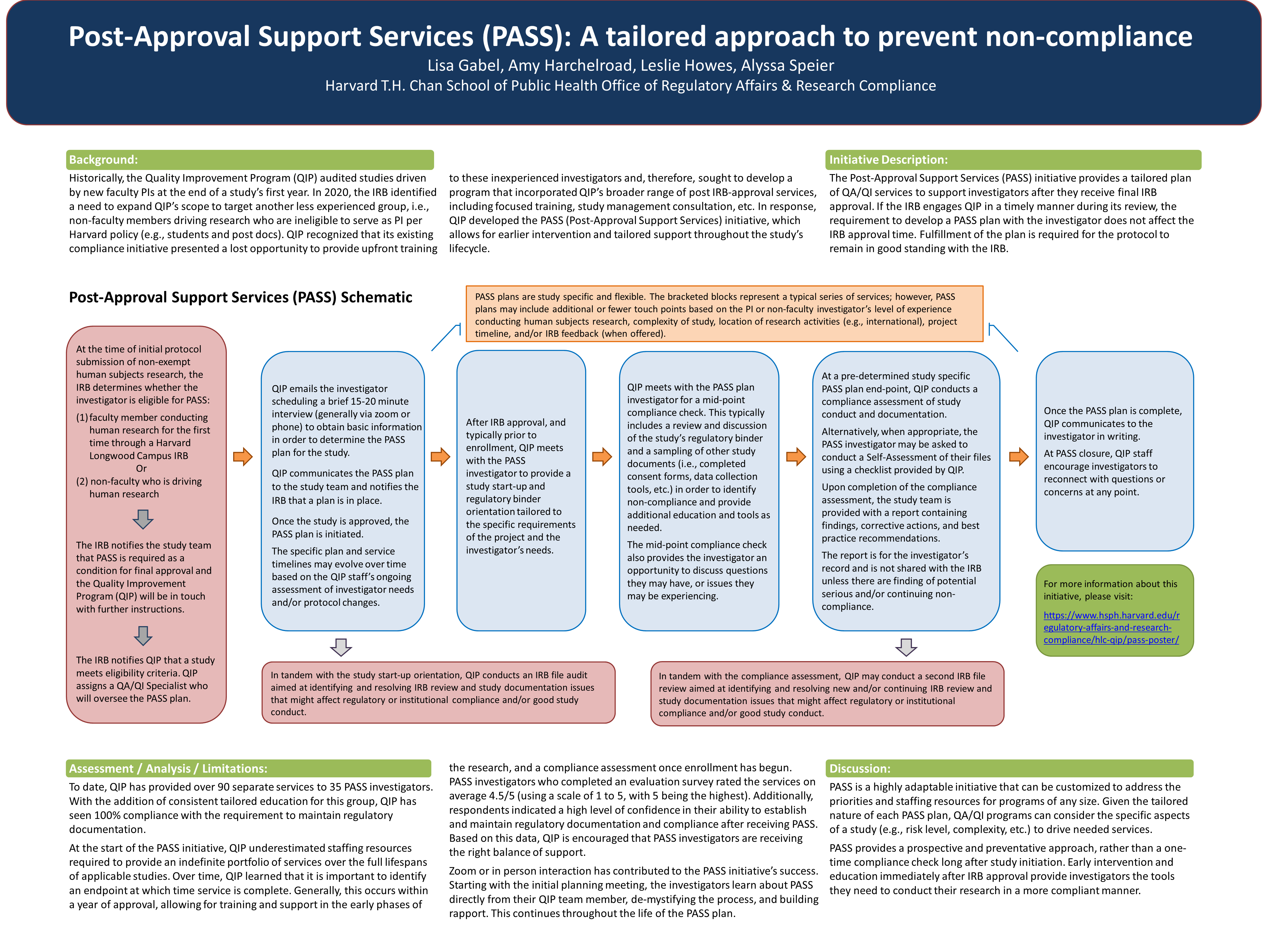
Background:
Historically, the Harvard Longwood Campus Schools[1] Quality Improvement Program (QIP) targeted a small subset of protocols (i.e., non-exempt human research with a first time PI) for a one-time audit at the end of the study’s first year. In 2020, the IRB identified a need to expand QIP’s scope to target another less experienced group, i.e., non-faculty members driving research who are ineligible to serve as PI per Harvard policy (e.g., students and post docs). QIP recognized that its existing compliance initiative presented a lost opportunity to provide upfront training to these inexperienced investigators and, therefore, sought to develop a program that incorporated QIP’s broader range of post IRB-approval services, including focused training, study management consultation, etc. In response, QIP developed the PASS (Post-Approval Support Services) initiative, which allows for earlier intervention and tailored support throughout the study’s lifecycle.
Program Description:
The Post-Approval Support Services (PASS) initiative provides a tailored plan of QA/QI services to support investigators after they receive final IRB approval. All eligible investigators are referred to the program and an anticipated plan for what the PASS services will consist of must be in place as a condition of IRB approval. If the IRB engages QIP in a timely manner during its review (i.e., soon after the submission is received), the requirement to develop a PASS plan with the investigator does not affect the IRB approval time. After approval is received, fulfillment of the plan is required in order for the protocol to remain in good standing with the IRB.
After IRB approval, QIP’s compliance activities also include an audit of the PASS affiliated IRB files. These compliance checks are aimed at identifying and resolving IRB review and study documentation issues that might impact regulatory or institutional compliance and good study conduct (e.g., appropriate waivers are in place, consent disclosures are consistent with the Research Protocol, provisions for a witness where required by regulatory or institutional policy are in place, etc.).
Assessment / Analysis / Limitations:
Under PASS, QIP has provided over 90 separate services to 35 investigators. With the addition of consistent tailored education for this group, since it’s initiation QIP has seen 100% compliance with the requirement for investigators to maintain regulatory documentation.
Discussion:
PASS is a highly adaptable initiative that can be customized to address the priorities and staffing resources for programs of any size. QIP has learned that its initial approach was unnecessarily general, but given the customizable nature of each PASS plan, QIP now considers the specific aspects of a study (e.g., risk level, complexity, etc.) to drive needed services.
While many compliance initiatives are reactive in their focus on answering the question of how to monitor studies after they have begun, PASS provides a prospective and preventative approach. Rather than conducting a one-time compliance check long after study initiation, QIP finds that providing early education and real-time assessment and intervention provides investigators the tools they need to conduct their research in a more compliant manner. QIP is confident that the knowledge gained by investigators while receiving their PASS services may also be applied to studies that they conduct in the future, leading to a more compliant generation of researchers.
For more information on the PASS initiative and/or QIP, please contact QIP@hsph.harvard.edu.
[1] Harvard Longwood Campus (HLC) Schools are comprised of Harvard T.H. Chan School of Public Health, Harvard Medical School, and Harvard School of Dental Medicine. The QIP program, housed at Harvard Chan School, serves the entire HLC schools research community.