In a paper published in the EMBO Journal in February, the Danial (Molecular Metabolism/ Dana Farber Cancer Institute) and Shirihai (UCLA) labs reported that fatty acid utilization is selectively coupled to mitochondrial morphology.
Increasing evidence indicates that changes in fuel utilization are associated with altered mitochondrial membrane morphology through fusion and fission events. However, the causality of the relationship between mitochondrial morphology and fuel metabolism has remained unclear.
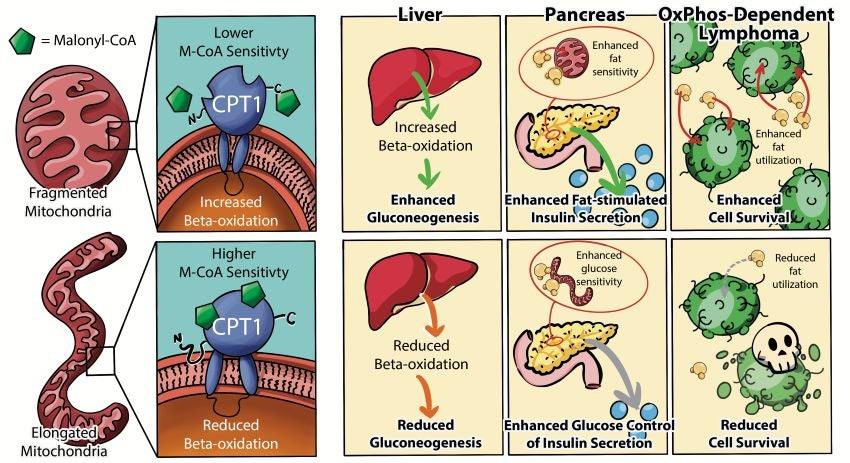
In this study, Molecular Metabolism/ Dana Farber Cancer Institute researchers and their collaborators at UCLA found that forced mitochondrial elongation by genetic manipulation selectively diminishes fatty acid oxidation, while forced fragmentation augments fatty acid oxidation without affecting the mitochondrial handling of other fuel substrates.
The findings indicate that the link between mitochondrial fragmentation and fatty acid oxidation is not only conserved across multiple cell types but is also required for specific fatty acid oxidation-driven functions. These biological consequences include gluconeogenesis in hepatocytes, adaptive increases in β-cell insulin secretion in response to obesity, and survival of fatty acid oxidation-dependent cancer cells.
The researchers found that the effect of the mitochondrial morphology on fatty acid oxidation is selective for long-chain fatty acids, pointing to a particular rate-limiting enzyme in fatty acid oxidation – CPT1 – as the downstream effector of mitochondrial architecture. The activity of CPT1 is inhibited by the elongation of mitochondria but enhanced by their fragmentation.
Overall, these findings point to a physiologic role for mitochondrial fragmentation as a basic mechanism for regulating cellular fuel preference for fatty acids.