In a paper published in JCI Insight, titled, “Endothelial FABP4 constitutes the majority of basal circulating hormone levels and regulates lipolysis-driven insulin secretion”, a team of researchers from the Hotamışlıgil Lab, Sabri Ülker Center, spearheaded by Karen Inouye and led by Gökhan Hotamışlıgil, demonstrated that the endothelium is the major source of circulating fatty acid binding protein-4 (FABP4), a lipid chaperone originally identified in adipocytes, with hormonal effects to regulate metabolism. Moreover, they showed that endothelial, rather than adipocyte, FABP4 is critical for pancreatic beta cell insulin responses to lipolysis.
High levels of circulating FABP4 are strongly associated with obesity and metabolic pathologies in experimental models and humans. As FABP4 is abundantly expressed in adipocytes and is secreted from adipocytes upon stimulation of lipolysis, researchers have assumed that adipocytes are the major source of circulating FABP4. Yet, this has not been confirmed in vivo.
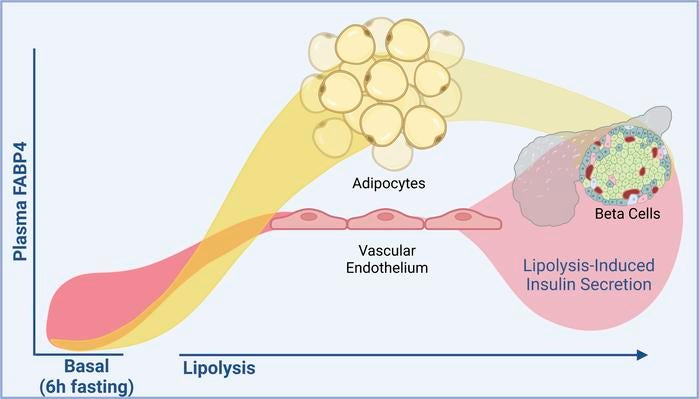
In this study, MET researchers generated a series of genetic mouse models lacking the Fabp4 gene in specific cell types. Unexpectedly, only 11% of baseline circulating plasma FABP4 in lean mice could be attributed to adipocytes. Instead, the experiments showed that endothelial cells – the cells that line our blood vessels – are the major contributor to baseline circulating FABP4, producing approximately 87% of total circulating FABP4 levels.
Further experiments showed that adipocytes are indeed the main source of increased plasma FABP4 levels during lipolysis. By contrast, mice lacking endothelial Fabp4 showed near-normal induction of FABP4 in response to lipolysis. Despite this, these mice exhibited reduced lipolysis-induced insulin responses, to the same degree as whole-body Fabp4 deletion mice. Thus, endothelial-derived FABP4 is critical for the insulin response to lipolysis.
Together, these findings implicate the endothelium as an endocrine organ and a potential key regulator of metabolism. Future work will determine the roles of endothelial versus adipocyte-derived FABP4 in obesity and other metabolic conditions.
Congratulations to Karen Inouye, Kacey Prentice, Alexandra Lee, Zeqiu Wang, Carla Dominguez-Gonzalez, Mu Xian Chen, Jillian Riveros, M. Furkan Burak, Grace Lee, and Gökhan Hotamışlıgil!
Read Full Publication Here


